how to draw molecular orbital diagram of no
For example B has two electrons in the 2s orbital and one in the 2p orbital. 8 - Drawing Molecular Orbital Diagrams.
Bonding And Antibonding Pi Orbitals Master Organic Chemistry
To check count how.

. First step is to determine which mo diagram were using. How to write the molecular orbital diagram for NO nitric oxide a heteronuclear molecule. Step 3 Fill in the electrons in the.
1a Draw the molecular orbital diagram of NO place the. How do you draw. Abstract TLDR Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already.
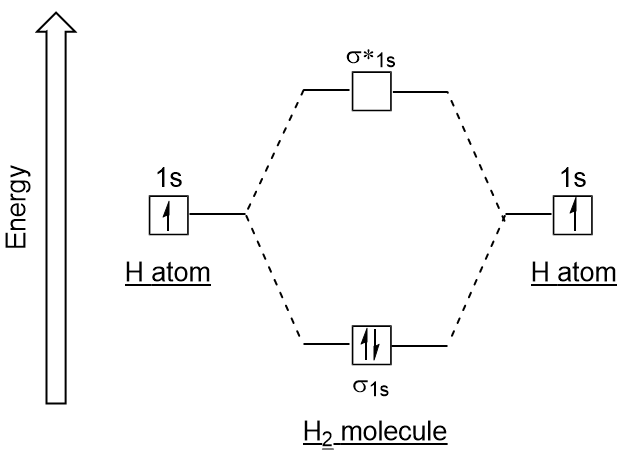
F has two electrons in the 2s energy level and five electrons in the 2p orbitals. Molecular orbital diagrams are complex involving two. 2 he2 has bond order 0 2 22 0 and we can make h.
Sorry the Sigma-1-s orbital is a little off-screen. So stability means lower in energy. In the molecular orbital diagram for the molecular ion n 2 the number of electrons in the σ 2 p molecular orbital is.
Youll get a detailed solution from a subject matter expert that helps you learn core concepts. This video discusses how to draw the molecular orbital MO diagram for the B2 molecule. I wouldnt violate the au.
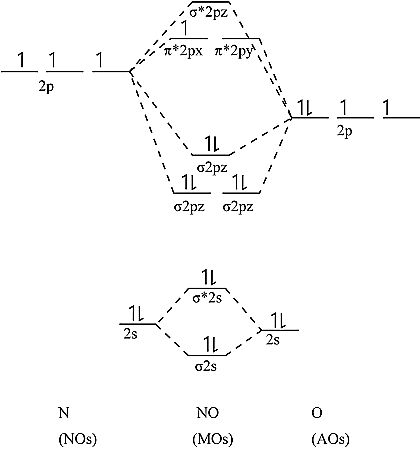
This problem has been solved. Electronic configuration of N atom is 1s2 2s2 2p3. The molecular orbital diagram has molecular orbital energy level at centre and is surrounded by atomic orbital energy level.
How to draw the molecular orbital diagram of O2. Fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals. MAKE SURE TO SUBSCRIBEThis video puts emphasis on molecular orbital diagrams a fundamental way of understanding why Diels-Alder chemistry works.
Draw the molecular orbital diagram for NO 3-Expert Solution. So lets go ahead and start with rule number one the simplest rule which says that the number of total molecular orbital energy States should be equal to the total number of atomic orbitals. Similar to atomic orbitals molecular orbitals are wave functions giving the probability of finding an electron in certain regions of a molecule.
According to molecular orbital theory. The bond order of the boron cation is also calculated and the mea. Keep in mind the energy of the atomic orbitals and molecular.
It shows electrons in both bonding and anti-bonding molecular. Rest assured its filled.

Molecular Orbital Diagram For No Download Scientific Diagram
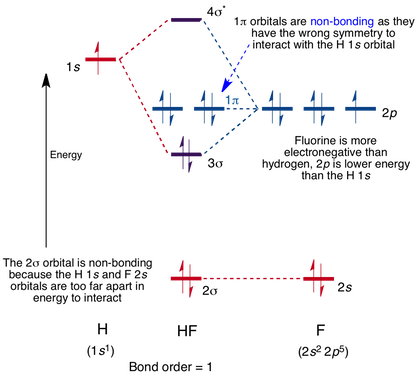
Mo For Hf Chemistry Libretexts

Chemical Bonding Molecular Orbitals Of H2 And He2 Britannica

8 Drawing Molecular Orbital Diagrams Flux Science
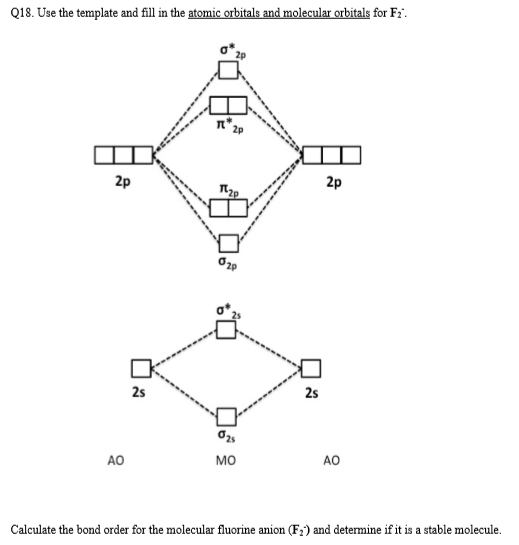
Solved Q20 Draw The Molecular Orbital Diagram For The Chegg Com

10 5 Molecular Orbital Theory Chemistry Libretexts

Write Mo Energy Label Diagram For Co And No Scholr

Introduction To Inorganic Chemistry Molecular Orbital Theory Wikibooks Open Books For An Open World
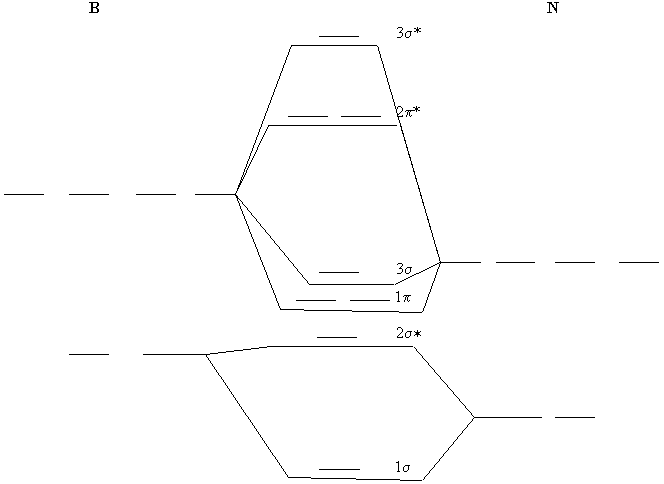
Molecular Structure Practice Problems Answers

The Pi Molecular Orbitals Of Benzene Master Organic Chemistry
Draw The Molecular Orbital Energy Level Diagram Of N 2 Molecules
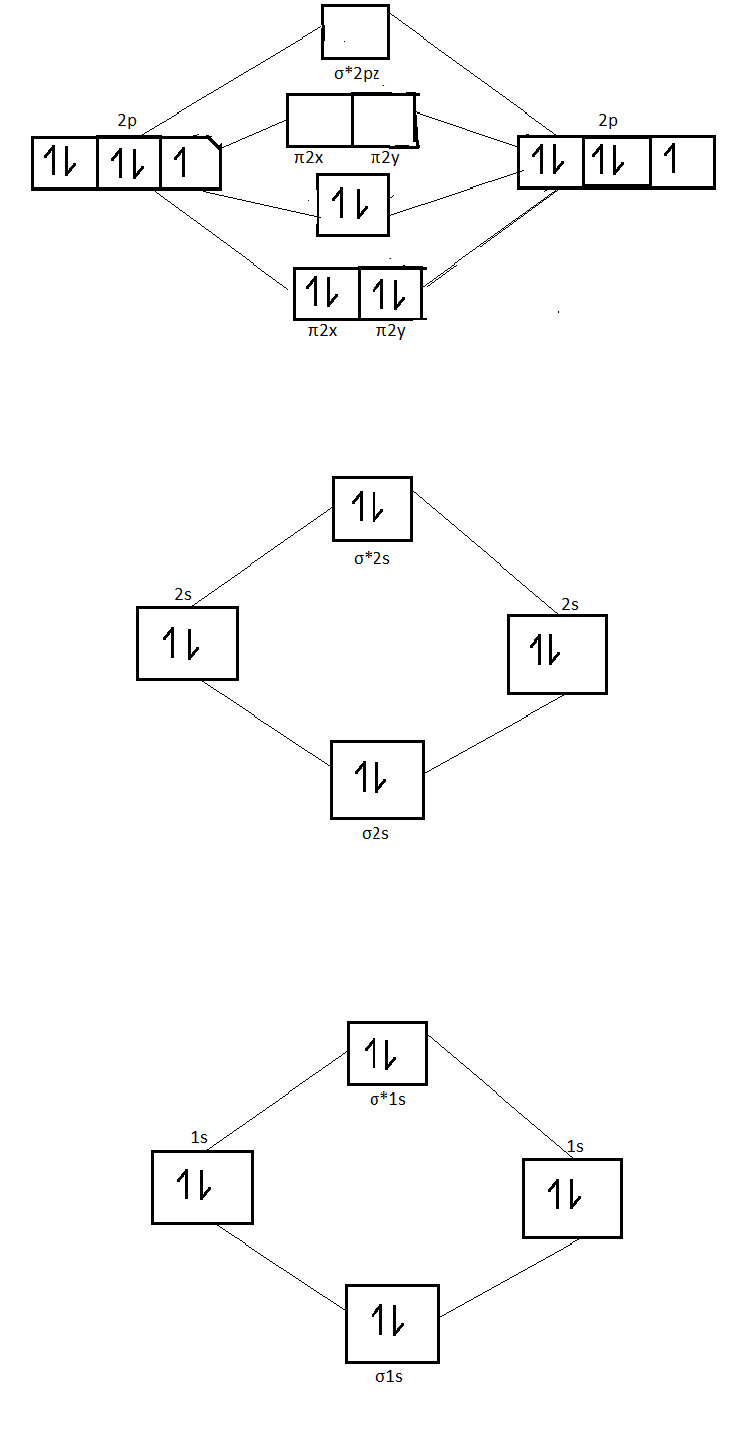
Using The Mo Diagram Of No Calculate The Bond Order Compare It To N O
Molecular Orbitals Introductory Chemistry 1st Canadian Edition

Orbital Diagram How To Draw Examples Rules Filling Order
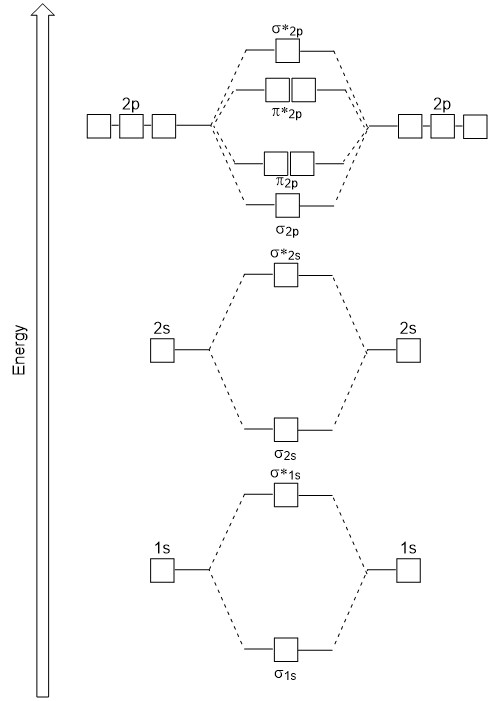
8 Drawing Molecular Orbital Diagrams Flux Science
Molecular Nitrogen And Related Diatomic Molecules
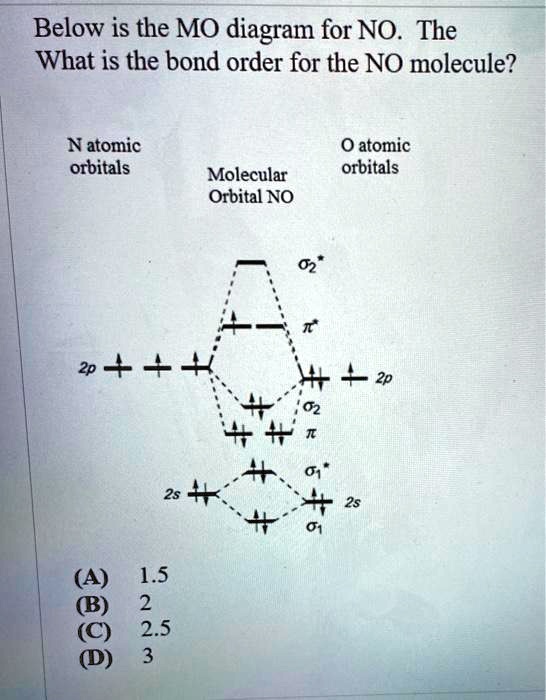
Solved Below Is The Mo Diagram For No The What Is The Bond Order For The No Molecule Natomic Orbitals 0 Atomic Orbitals Molecular Orbital No 2 Zp 4 1 5 8 2 2 5

Draw The Molecular Orbital Energy Diagram For Oxygen Molecule O2 And Show That I It Has A Double Bond Ii It Has Paramagnetic Character From Chemistry Chemical Bonding And Molecular Structure Class 11 Cbse

How To Draw Molecular Orbital Diagram Best Online Free Chemistry Learning